cMRI
Equipment
7 Tesla MRI
<7.0 Tesla MRI (Discovery MR950, GE Healthcare)>
Multiphoton microscopy
<Zeiss LSM510 META NLO laser scanning microscope>
Multiphoton microscopy using a fentsecond laser of the near infrared spectral range (between 700 and 1100 nm), in ultrashort pulses with single pulse energy of up to 170 kW and a repetition rate of 76 to 90 MHz, can excite fluorescence of the spot-focused on the object of interest if at least two photons are absorbed by a fluorochrome molecule within less than a femtosecond (10–15 seconds). The spatial confinement of multiphoton excitation gives rise to several advantages over single-photon confocal microscopy, especially at the aspects of increasing penetration depth of the excitation beam and markedly reducing overall photobleaching and photodamage. Properly applied, it is capable of measuring calcium transients 500 μm deep in a mouse brain, or quantifying blood flow by imaging shadows of blood cells as they race through capillaries.
Our institute has Zeiss LSM510 META NLO laser scanning microscope equipped with a Spectra-Physics Mai Tai Ti:sapphire laser. We use this system for observing the process of vessel formation during the embryogenesis of transgenic zebrafishes and mice of which endothelial cells expressing fluoroprobes by long-term live-imaging. Potential applications of the multiphoton laser scanning microscopy as applied to integrative vascular physiology of the brain are also strongly expected.
Ultracryo thin sectioning system
<EM-UC6 / EM-FC6 LEICA>
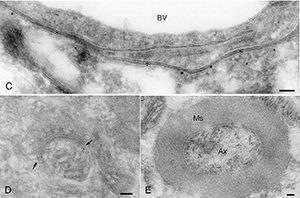
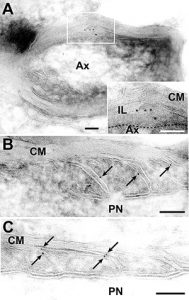
This device provide us 70-90nm-thick ultrathin sections of frozen tissue under freezing temperature (-120 degrees C). Since these sections are obtained without dehydration and resin-embedded processes, they are most suitable for immunogold labeling and EM analysis. Immunogold labeling on ultracryo thin sections would be the ‘state of the art’ way to analyze molecular localization at the nano-meter resolution (Fig. 1, 2)
Fig. 1: GGLAST in astrocyte endfeet (C); mGluR1 in synapse (D); neurofilament protein in axon (E). [J Neurosci Methods 153: 2. 276-282, 2006]
Fig. 2: Transmembrane sialylglycoprotein, opalin, localize at loops of oligodendrocyte in CNS (A-C). [J Biol Chem 283: 30. 20830-20840, 2008]
Rapid freezing equipment for liquid helium
<HIF-4K Hitachi>
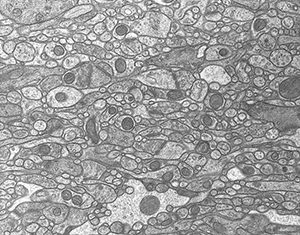
Freeze-substitution method would be one of the best procedures to minimize artifacts during processing biological specimens. In this method, lower freezing temperature brings us better preservation of ultrastructure. In this equipment, fresh specimens from living animals or culture cells are frozen ultra short period at liquid helium temperature, -260 degrees C. Combined with substitution process, we will be able to observe well-preserved ultrastructure without several artifacts (Fig. 3).
Fig. 3: An EM photograph of cerebellar cortex. Freeze-substitution using liquid He.